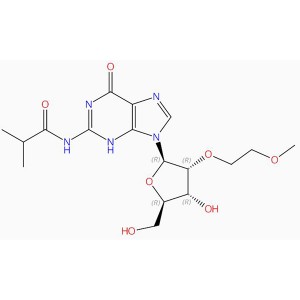
C17H25N5O7 Guanosine, 2′-O-(2-methoxyethyl)-N-(2-methyl-1-oxopropyl)- (9CI, A CI)
| Key Physical Properties | Value | Condition |
| Molecular Weight | 411.41 | - |
| Melting Point (Experimental) | 137-139.2 °C | - |
| Density (Predicted) | 1.60±0.1 g/cm3 | Temp: 20 °C; Press: 760 Torr |
| pKa (Predicted) | 8.68±0.20 | Most Acidic Temp: 25 °C |
Canonical SMILES O=C1N=C(NC(=O)C(C)C)NC2=C1N=CN2C3OC(CO)C(O)C3OCCOC
Isomeric SMILES O=C1C2=C(N(C=N2)[C@H]3[C@H](OCCOC)[C@H](O)[C@@H](CO)O3)NC(NC(C(C)C)=O)=N1
InChI
InChI=1S/C17H25N5O7/c1-8(2)14(25)20-17-19-13-10(15(26)21-17)18-7-22(13)16-12(28-5-4-27-3)11(24)9(6-23)29-16/h7-9,11-12,16,23- 24H,4-6H2,1-3H3,(H2,19,20,21,25,26)/t9-,11-,12-,16-/m1/s1
InChI Key
IZOOGJIUOCHAAY-UBEDBUPSSA-N
1 Other Name for this Substance
2′-O-(2-Methoxyethyl)-N-(2-methyl-1-oxopropyl)guanosine (ACI)
| Properties available |
| Thermal |
Thermal
| Property | Value | Condition | Source |
| Melting Point | 137-139.2 °C | (1) CAS | |
(1) Taj, Shabbir Ali S.; Nucleosides, Nucleotides & Nucleic Acids, (2008), 27(9), 1024-1033, CAplus
Spectra available
1H NMR
13C NMR
IR
| Properties available |
| Biological |
| Chemical |
| Density |
| Lipinski |
| Structure Related |
Biological
| Property | Value | Condition | Source |
| Bioconcentration Factor | 1.0 | pH 1; Temp: 25 °C | (1) ACD |
| Bioconcentration Factor | 1.0 | pH 2; Temp: 25 °C | (1) ACD |
| Bioconcentration Factor | 1.0 | pH 3; Temp: 25 °C | (1) ACD |
| Bioconcentration Factor | 1.0 | pH 4; Temp: 25 °C | (1) ACD |
| Bioconcentration Factor | 1.0 | pH 5; Temp: 25 °C | (1) ACD |
| Bioconcentration Factor | 1.0 | pH 6; Temp: 25 °C | (1) ACD |
| Bioconcentration Factor | 1.0 | pH 7; Temp: 25 °C | (1) ACD |
| Bioconcentration Factor | 1.0 | pH 8; Temp: 25 °C | (1) ACD |
| Bioconcentration Factor | 1.0 | pH 9; Temp: 25 °C | (1) ACD |
| Bioconcentration Factor | 1.0 | pH 10; Temp: 25 °C | (1) ACD |
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs)
Chemical
| Property | Value | Condition | Source |
| Koc | 1.0 | pH 1; Temp: 25 °C | (1) ACD |
| Koc | 1.01 | pH 2; Temp: 25 °C | (1) ACD |
| Koc | 5.81 | pH 3; Temp: 25 °C | (1) ACD |
| Koc | 11.4 | pH 4; Temp: 25 °C | (1) ACD |
| Koc | 12.6 | pH 5; Temp: 25 °C | (1) ACD |
| Koc | 12.7 | pH 6; Temp: 25 °C | (1) ACD |
| Koc | 12.5 | pH 7; Temp: 25 °C | (1) ACD |
| Koc | 10.6 | pH 8; Temp: 25 °C | (1) ACD |
| Koc | 4.20 | pH 9; Temp: 25 °C | (1) ACD |
| Koc | 1.0 | pH 10; Temp: 25 °C | (1) ACD |
| logD | -2.44 | pH 1; Temp: 25 °C | (1) ACD |
| logD | -1.60 | pH 2; Temp: 25 °C | (1) ACD |
| logD | -0.84 | pH 3; Temp: 25 °C | (1) ACD |
| logD | -0.55 | pH 4; Temp: 25 °C | (1) ACD |
| logD | -0.50 | pH 5; Temp: 25 °C | (1) ACD |
| logD | -0.50 | pH 6; Temp: 25 °C | (1) ACD |
| logD | -0.51 | pH 7; Temp: 25 °C | (1) ACD |
| logD | -0.58 | pH 8; Temp: 25 °C | (1) ACD |
| logD | -0.98 | pH 9; Temp: 25 °C | (1) ACD |
| logD | -1.76 | pH 10; Temp: 25 °C | (1) ACD |
| logP | -0.497±0.633 | Temp: 25 °C | (1) ACD |
| Mass Intrinsic Solubility | 0.58 g/L | Temp: 25 °C | (1) ACD |
| Mass Solubility | 49 g/L | pH 1; Temp: 25 °C | (1) ACD |
| Mass Solubility | 7.0 g/L | pH 2; Temp: 25 °C | (1) ACD |
| Mass Solubility | 1.2 g/L | pH 3; Temp: 25 °C | (1) ACD |
| Mass Solubility | 0.62 g/L | pH 4; Temp: 25 °C | (1) ACD |
| Mass Solubility | 0.58 g/L | pH 5; Temp: 25 °C | (1) ACD |
| Mass Solubility | 0.58 g/L | pH 6; Temp: 25 °C | (1) ACD |
| Mass Solubility | 0.58 g/L | pH 7; Temp: 25 °C | (1) ACD |
| Mass Solubility | 0.70 g/L | pH 8; Temp: 25 °C | (1) ACD |
| Mass Solubility | 1.7 g/L | pH 9; Temp: 25 °C | (1) ACD |
| Mass Solubility | 10 g/L | pH 10; Temp: 25 °C | (1) ACD |
| Property | Value | Condition | Source |
| Mass Solubility | 0.58 g/L | Unbuffered Water pH 5.98; Temp: 25 °C | (1) ACD |
| Molar Intrinsic Solubility | 1.4 x 10-3 mol/L | Temp: 25 °C | (1) ACD |
| Molar Solubility | 0.12 mol/L | pH 1; Temp: 25 °C | (1) ACD |
| Molar Solubility | 0.017 mol/L | pH 2; Temp: 25 °C | (1) ACD |
| Molar Solubility | 3.0 x 10-3 mol/L | pH 3; Temp: 25 °C | (1) ACD |
| Molar Solubility | 1.5 x 10-3 mol/L | pH 4; Temp: 25 °C | (1) ACD |
| Molar Solubility | 1.4 x 10-3 mol/L | pH 5; Temp: 25 °C | (1) ACD |
| Molar Solubility | 1.4 x 10-3 mol/L | pH 6; Temp: 25 °C | (1) ACD |
| Molar Solubility | 1.4 x 10-3 mol/L | pH 7; Temp: 25 °C | (1) ACD |
| Molar Solubility | 1.7 x 10-3 mol/L | pH 8; Temp: 25 °C | (1) ACD |
| Molar Solubility | 4.2 x 10-3 mol/L | pH 9; Temp: 25 °C | (1) ACD |
| Molar Solubility | 0.025 mol/L | pH 10; Temp: 25 °C | (1) ACD |
| Molar Solubility | 1.4 x 10-3 mol/L | Unbuffered Water pH 5.98; Temp: 25 °C | (1) ACD |
| Molecular Weight | 411.41 | ||
| pKa | 8.68±0.20 | Most Acidic Temp: 25 °C | (1) ACD |
| pKa | 3.05±0.20 | Most Basic Temp: 25 °C | (1) ACD |
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs)
Density
| Property | Value | Condition | Source |
| Density | 1.60±0.1 g/cm3 | Temp: 20 °C; Press: 760 Torr | (1) ACD |
| Molar Volume | 256.2±7.0 cm3/mol | Temp: 20 °C; Press: 760 Torr | (1) ACD |
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs)
Lipinski
| Property | Value | Condition | Source |
| Freely Rotatable Bonds | 9 | (1) ACD | |
| H Acceptors | 12 | (1) ACD | |
| H Donors | 4 | (1) ACD | |
| H Donor/Acceptor Sum | 16 | (1) ACD | |
| logP | -0.497±0.633 | Temp: 25 °C | (1) ACD |
| Molecular Weight | 411.41 |
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs)
Structure Related
| Property | Value |
Condition |
Source |
| Polar Surface Area | 157 A2 | (1) ACD | |
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs)
Spectra available
1H NMR
13C NMR



![C21H23N3O5 L-Ornithine, N5-(aminocarbonyl)-N2-[(9H-fluoren-9-ylmethoxy) carbonyl]- (9CI, ACI)](https://cdn.globalso.com/nvchem/C21H23N3O5-L-Ornithine-300x300.jpg)
![C20H31NO5 Heptanoic acid, 3- hydroxy-5-methyl-4-[[(phenylmethoxy)carbonyl] amino]-, 1,1-dimethylethyl ester, [3R-(3R*,4S*,5S*)]- (9CI) H301](https://cdn.globalso.com/nvchem/C20H31NO5-Heptanoic-acid-300x300.jpg)
![C50H58N7O9P Adenosine, N-benzoyl-5′ -O- [bis(4-methoxyphenyl)phenylmethyl]-2′ – O-(2-methoxyethyl)-, 3′ – [2-cyanoethyl N,N-bis(1-methylethyl) phosphoramidite] (ACI)](https://cdn.globalso.com/nvchem/C50H58N7O9P-Adenosine-300x300.png)
![C41H39NO6 1-Pyrrolidinecarboxylic acid, 2-[[bis(4-methoxyphenyl)phenylm ethoxy]methyl]-4-hydroxy-, 9H-fluoren-9-ylmethyl ester, (2S,4R)- (9 CI, ACI)](https://cdn.globalso.com/nvchem/C41H39NO6-1-Pyrrolidinecarboxylic-acid-300x300.jpg)
![C21H21N3O6 Thymidine, α – [(1-naphthalenylmethyl)amino]- α -oxo- (ACI)](https://cdn.globalso.com/nvchem/C21H21N3O6-Thymidine-300x300.png)